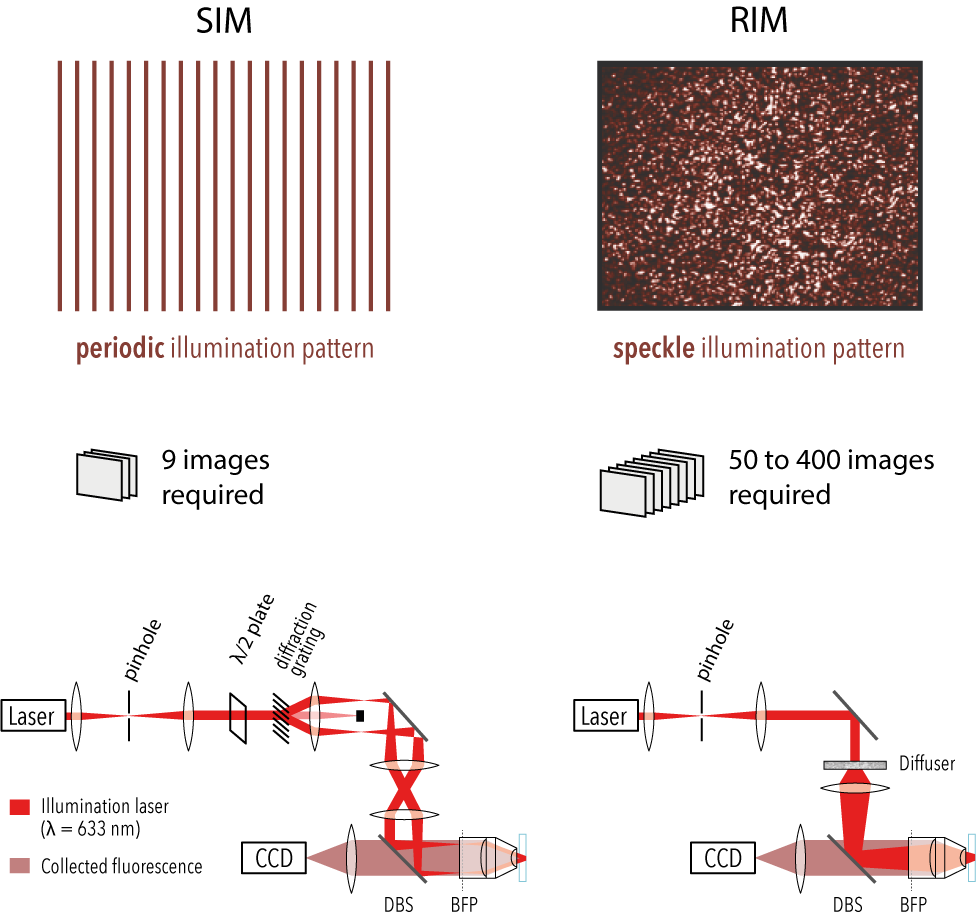
SIM
Widefield fluorescence microscopy is a key tool in biology because of its ability to provide functional images of living samples over large fields of view. However, its lateral resolution is diffraction limited to around 200 nm and the absence of optical sectioning results in a strong out-of-focus background signal which degrades the image contrast.
A widespread solution for improving the resolution while keeping the speed and low phototoxicity required for live imaging, is Structured Illumination Microscopy (SIM). SIM reconstructs numerically the super-resolved image of the sample from several (less than ten in standard two-dimensional SIM) low-resolution images recorded under translated and rotated periodic illuminations. The structured illumination acts as a carrier signal which down-modulates sample high spatial frequencies into the pass-band of the microscope. In the last twenty years, SIM has been implemented with many different patterns, 1D light grids, 2D periodic spots, hexagonal lattice, and has provided spectacular images of live specimen.
Its main issue lays in the reconstruction (or demodulation) procedure which requires the knowledge and thus the control of each illumination pattern. Unwanted pattern distortion, caused by the sample itself or by slight misalignements, drifts, or optics’ aberrations yield loss in resolution and image artefacts. As a result, SIM is not adapted to the imaging of thick of aberrating samples. In addition, its experimental implementation is difficult and it must be operated by experts.
blind-SIM
To get round the crucial issue of the knowledge and control of the illuminations in a radical way, we proposed to illuminate the sample with random speckled patterns (obtained by passing the laser beam through a diffuser) and to use only the statistical property of the speckles in the reconstruction [1]. The speckle statistics are robust to scattering and aberrations, which relaxes the constraints on the illuminations.
The first reconstruction schemes used only the first moment of the speckles (their uniform average) [1]. In the simplest approach, the super-resolved image was formed from the average of the deconvolved low-resolution speckled images under sparsity or positivity constraints [2]. The resolution gain stemmed from the sparsity regularization that was activated by the speckled illumination [3].
This work laid the foundations for a broader and more versatile technique that we later developed, called RIM (Random Illumination Microscopy, see next section).
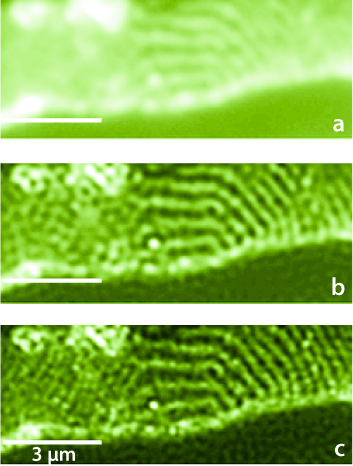 |
Figure 1: 80-nm thick Epon-embedded section of rabbit jejunum with glycoproteins marked with Cy5 fluorescent dye. It is illuminated by 150 different random speckle patterns through an oil objective (NA = 1.45) at λ = 633 nm. a Wide-field image of the sample. b Deconvolution of the wide-field image in (a). c Blind-SIM fluorescence density obtained from the 150 speckle images. Reproduced from Ref. [1]. |
RIM
In a more recent method, called Random Illumination Microscopy (RIM), we proposed to use the second order statistics of the SIM speckles. The super-resolved image was estimated from the variance of the speckled images using an iterative variance (or standard deviation) matching algorithm (algoRIM) [4,5,9]. The latter required only the knowledge of the speckles auto-covariance, which is well-known theoretically. Remarkably, even though taking the variance of the speckled images is a non-linear data processing, it was demonstrated mathematically and shown experimentally that RIM super-resolved reconstruction was linearly linked to the sample fluorescence density with a lateral resolution gain comparable to SIM [4,5,9]. In addition, it exhibited an optical sectioning comparable to that of an ideal confocal [5].
Using random speckled illuminations and variance data processing simplify drastically the experiment and the numerical reconstruction. RIM has been shown to produce robust super-resolved imaging of live and fixed biological samples even in difficult configurations [5,6,8] and was recently extended to non-linear imaging [7] (see next sections).
RIM is unaffected by optical aberrations on the excitation side, linear to brightness, and compatible with multicolor live-cell imaging over extended periods of time. We illustrate the potential of RIM on diverse biological applications, from the mobility of proliferating cell nuclear antigen (PCNA) in U2OS cells and kinetochore dynamics in mitotic S. pombe cells to the 3D motion of myosin minifilaments deep inside Drosophila tissues.
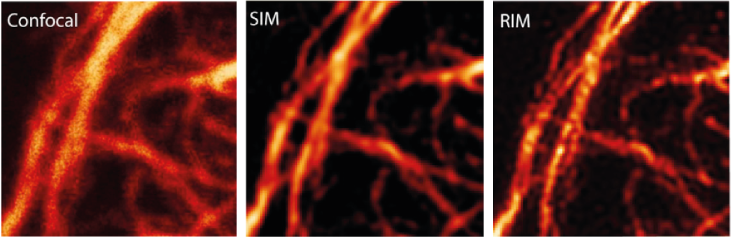 |
Images of the same vimentin network from fixed HUVEC cell with, confocal, SIM and RIM, with excitation at 561 nm and emission at 700 nm (large Stokes shift), NA = 1.4. The RIM image is better than the SIM reconstruction. Reproduced from [5]. |
TIRF-RIM
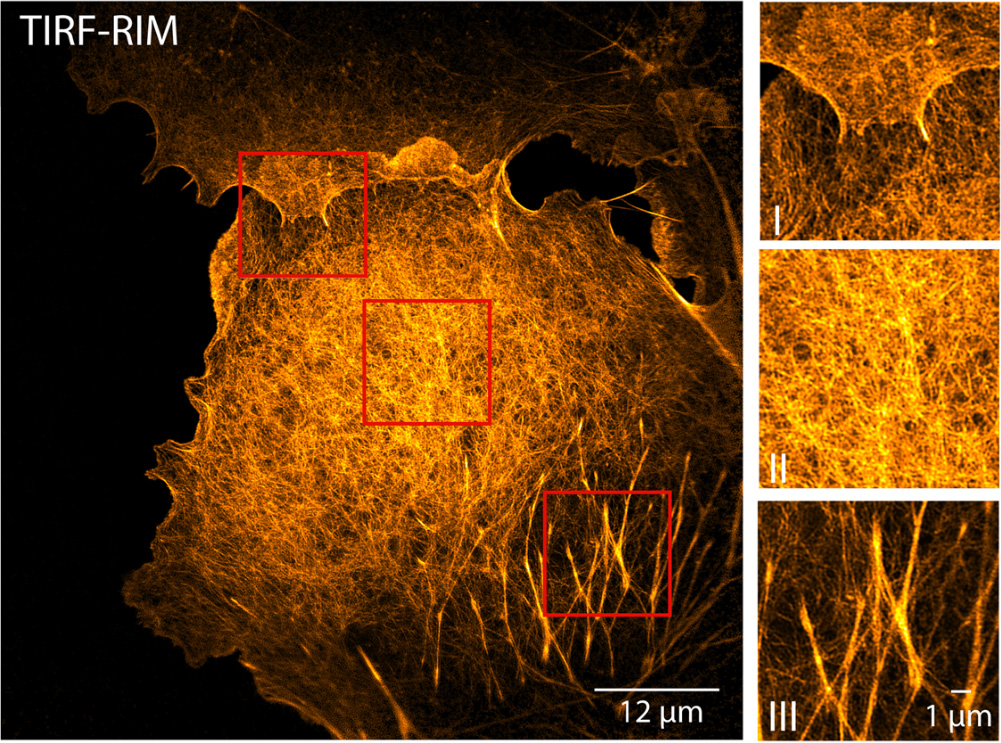
We implemented RIM in the total internal reflection fluorescence (TIRF) configuration for imaging biological processes close to the coverslip surface [6]. Using standard TIRF objectives, we separated fluorescent lines 60 nm apart and achieved high contrast 86 nm resolution on fixed biological samples. Applied to live macrophages, TIRF-RIM provided two-color dynamic images of paxillin nanoclusters with remarkable spatial (96–120 nm) and temporal (1–8 Hz) resolutions, respectively. The simple experimental setup and imaging protocol together with the robustness of the data processing to leaks and aberrations make TIRF-RIM a method of choice for super-resolution TIRF imaging.
 |
Comparison of TIRF-RIM and TIRF-SIM on images of fixed COS-7 cells labeled for actin. Reproduced from Ref. [6]. |
CARS-RIM
Coherent Raman microscopy is the method of choice for the label-free, real-time characterization of the chemical composition in biomedical samples. The common implementation relies on scanning two tightly focused laser beams across the sample, which frequently leads to sample damage and proves slow over large fields of view. The few existing wide-field techniques, for their part, feature a reduced lateral resolution and do not provide axial sectioning. To resolve these practical limitations, in collaboration with the group of Hervé Rigneault, we developed a robust wide-field nonlinear microscope that combines random illumination microscopy (RIM) with coherent anti-Stokes Raman scattering (CARS) and sum-frequency generation (SFG) contrasts [7].
Based on a comprehensive theoretical study, CARS-RIM provides super-resolved reconstructions and optical sectioning of the sample from the second-order statistics of multiple images obtained under different speckled illuminations. We experimentally show that multimodal CARS-RIM and SFG-RIM achieve wide-field nonlinear imaging with a 3 μm axial sectioning capability and a 300 nm transverse resolution, effectively reducing the peak intensity at the sample compared with conventional point-scanning CARS. We exemplify the label-free, highly contrasted chemical imaging potential of CARS-RIM and SFG-RIM wide-field microscopy in two dimensions, as well as three dimensions, for a variety of samples such as beads, unstained human breast tissue and a mixture of chemical compounds.
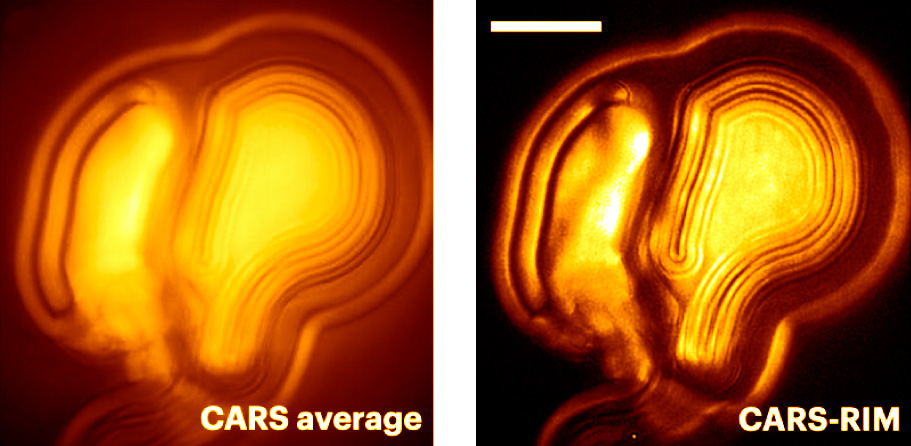 |
Multilamellar vesicles imaged with CARS and CARS-RIM at 2,850 cm−1. Reproduced from Ref. [7]. |
EDF-RIM
The ultimate aim of fluorescence microscopy is to achieve high-resolution imaging of increasingly larger biological samples. Extended depth of field presents a potential solution to accelerate imaging of large samples when compression of information along the optical axis is not detrimental to the interpretation of images. We have implemented an extended depth of field (EDF) approach in a random illumination microscope (RIM) [8]. RIM uses multiple speckled illuminations and variance data processing to double the resolution. It is particularly adapted to the imaging of thick samples as it does not require the knowledge of illumination patterns. We demonstrate highly-resolved projective images of biological tissues and cells. Compared to a sequential scan of the imaged volume with conventional 2D-RIM, EDF-RIM allows an order of magnitude improvement in speed and light dose reduction, with comparable resolution. As the axial information is lost in an EDF modality, we propose a method to retrieve the sample topography for samples that are organized in cell sheets.
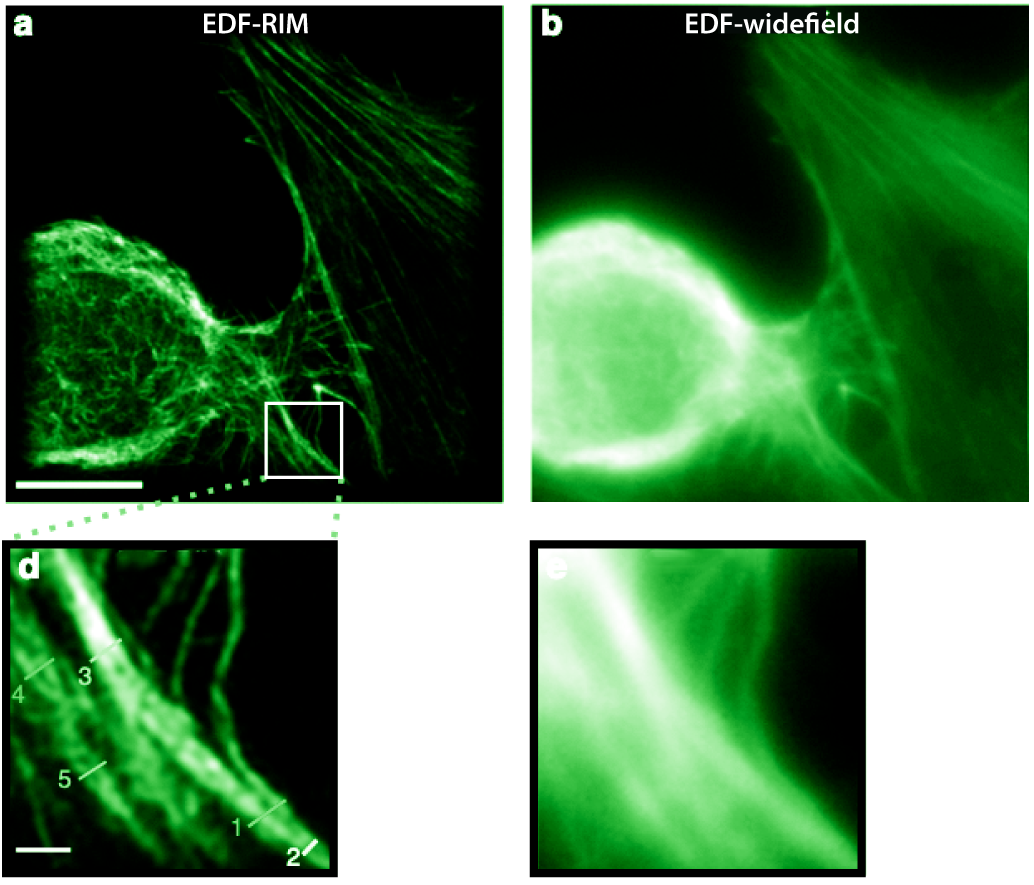 |
Phalloidin-alexa488 labeling of the actin cytoskeleton on a cultured cell imaged with (a) EDF-RIM and EDF-widefield. Reproduced from Ref. [8]. |
References
- [9] Fast super-resolved reconstructions in fluorescence random illumination microscopy (RIM)
G. Giroussens, S. Labouesse, M. Allain, T. Mangeat, L. Mazzella, L. Legoff, A. Sentenac, J. Idier
IEEE Transactions on Computational Imaging (2025) - [8] Extended-depth of field random illumination microscopy, EDF-RIM, provides super-resolved projective imaging
L. Mazzella, T. Mangeat, G. Giroussens, B. Rogez, H. Li, J. Creff, M. Saadaoui, C. Martins, R. Bouzignac, S. Labouesse, J. Idier, F. Galland, M. Allain, A. Sentenac, L. LeGoff
Light: Science & Applications 13, 285 (2024) - [7] Wide-field coherent anti-Stokes Raman scattering microscopy using random illuminations
E. M. Fantuzzi, S. Heuke, S. Labouesse, D. Gudavicius, R. Bartels, A. Sentenac, H. Rigneault
Nature Photonics 17, 1097–1104 (2023) - [6] Super-resolved total internal reflection fluorescence microscopy using random illuminations
K. Affannoukoué, S. Labouesse, G. Maire, L. Gallais, J. Savatier, M. Allain, M. Rasedujjan, L. Legoff, J. Idier, R. Poincloux, F. Pelletier, C. Leterrier, T. Mangeat, A. Sentenac
Optica 10, 1009 (2023) - [5] Super-resolved live-cell imaging using random illumination microscopy
T. Mangeat, S. Labouesse, M. Allain, A. Negash, E. Martin, A. Guénolé, R. Poincloux, C. Estibal, A. Bouissou, S. Cantaloube, E. Vega, T. Li, C. Rouvière, S. Allart, D. Keller, V. Debarnot, X. Bo Wang, G. Michaux, M. Pinot, R. Le Borgne, S. Tournier, M. Suzanne, J. Idier, A. Sentenac
Cell Reports Methods 1, 100009 (2021) - [4] On the Superresolution Capacity of Imagers Using Unknown Speckle Illuminations
J. Idier, S. Labouesse, M. Allain, P. Liu, S. Bourguignon
IEEE Transactions on Computational Imaging 4, 87-98 (2017) - [3] Joint reconstruction strategy for structured illumination microscopy with unknown illuminations
S. Labouesse, A. Negash, J. Idier, S. Bourguignon, T. Mangeat, P. Liu, A. Sentenac, M. Allain
IEEE Transactions on Image Processing 26, 2480-2493 (2017)
- [2] Improving the axial and lateral resolution of three-dimensional fluorescence microscopy using random speckle illuminations
A. Negash, S. Labouesse, N. Sandeau, M. Allain, H. Giovannini, J. Idier, R. Heintzmann, P. C. Chaumet, K. Belkebir, A. Sentenac
JOSA A 33, 1089 (2016) - [1] Structured illumination microscopy using unknown speckle patterns
E. Mudry, K. Belkebir, J. Girard, J. Savatier, E. Le Moal, C. Nicoletti, M. Allain, A. Sentenac
Nature Photonics 6, 312 (2012)

